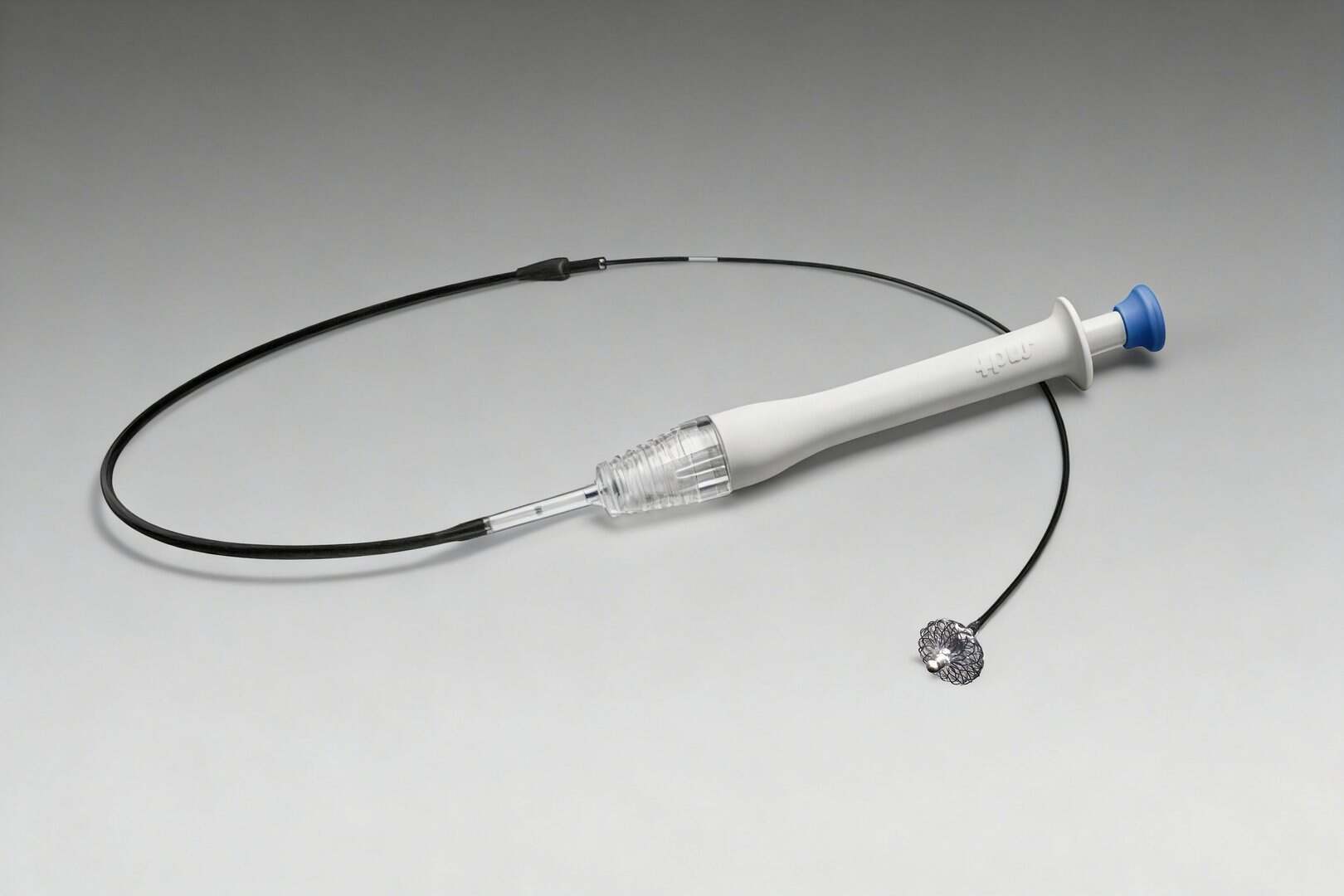
Swift Ultima
- Next generation Collabond™ technology endows collagen sponge with adhesive capacity and further thrombogenic ability.
Next-generation embolization device for endovascular occlusion (artery/vein, peripheral/neurovascular)
Significant advantage over coils, plugs, fluid embolics, and microspheres
Engineered for delivery through catheter, and only expands outside of catheter
Indicated to occlude arteries, veins or aneurysms
Indicated to be performed under fluoroscopy or ultrasound guidance
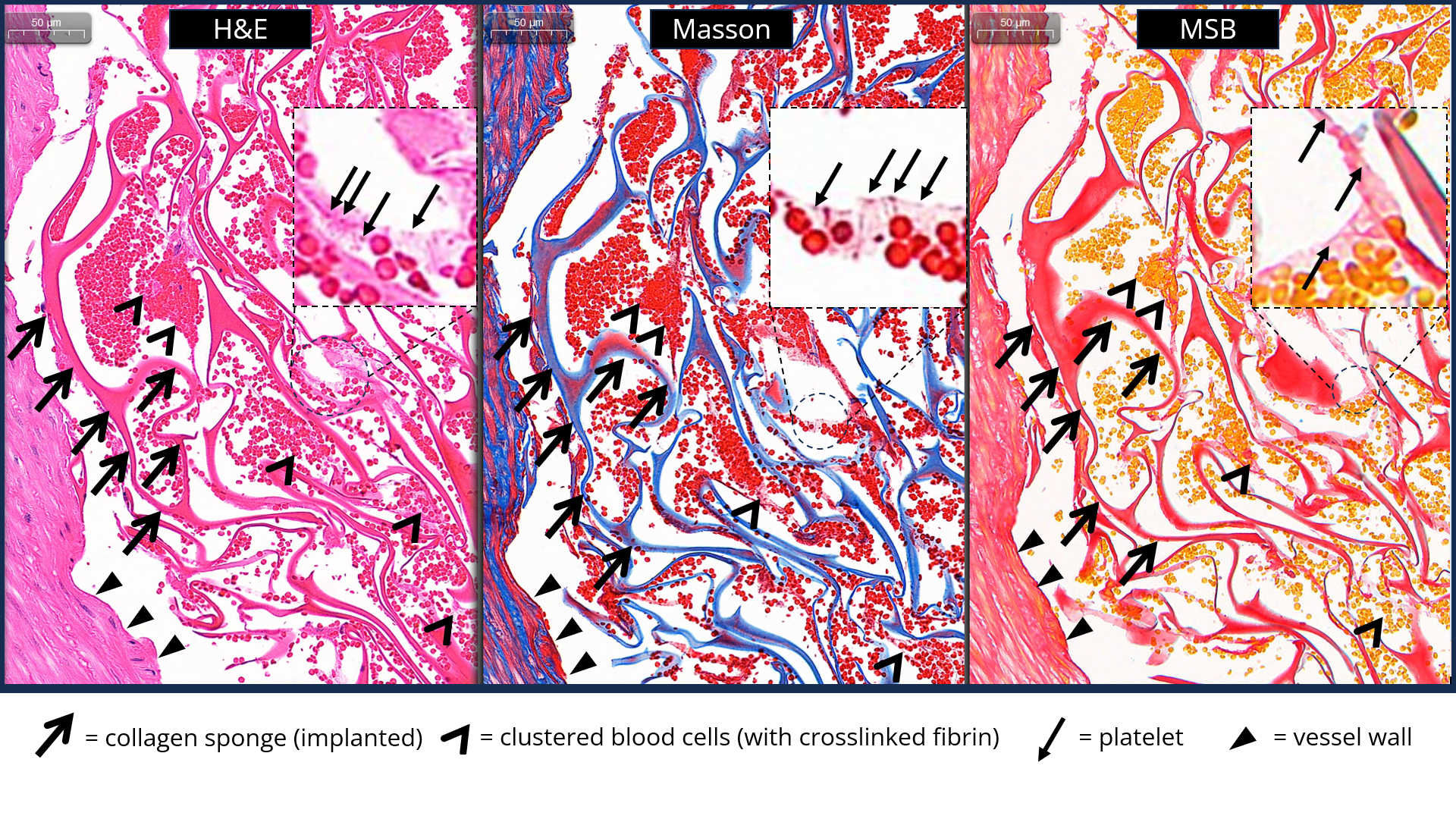
Expansible collagen sponge enhances compact occlusion filling the whole cavity
Collagen initiates hemostasis rapidly
Histology analysis shows rapid thrombus formation immediately after implantation(as the left figure shows)
*The National Medical Products Administration (NMPA), formerly known as the CFDA, is China’s regulator for drugs, devices and cosmetics.



A novel esophageal retraction balloon catheter, designed for esophageal protection during radiofrequency ablation for atrial fibrillation.
Now in commercial use, Safer supports both transoral and transnasal insertion pathways and has already been adopted in a growing number of clinical centers.
Demonstrated excellent performance in hundreds of conscious‑sedation procedures, reinforcing its effectiveness and ease of use.
Novel ultrasound system for super-selecting challenging vasculature under direct intravascular ultrasound guidance.
Indications such as in situ fenestration for aortic endografts and adrenal venous sampling (AVS) are under development.